Survival rates for cardiac arrest outside of a hospital setting are poor. Only about 5% of people survive, though encouraging people to get trained in cardio-pulmonary resuscitation (CPR) and the placement of defibrillators in public places like sports and recreation facilities have improved the situation somewhat.

For those individuals that do survive, brain damage can have serious effects that may not be reversible. This makes preventing brain injury in the first place a top priority. A team at the Ottawa Heart Institute is working to further improve survival rates and outcomes for cardiac arrest patients who make it to the hospital, through its Regional Cardiac Arrest Program—the first of its kind in Canada.
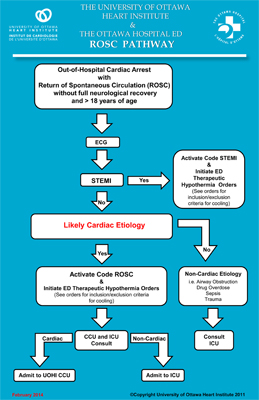
The operative phrase is “Code ROSC.” ROSC stands for “return of spontaneous circulation.” It means that the heart of someone who has gone into cardiac arrest is now beating on its own. When used, a Code ROSC alert sets off a process that sees cardiac arrest patients transferred to the Heart Institute as soon as they are stabilized in the peripheral hospital emergency department.
The program and the Code ROSC protocol are built on the model of Heart Institute’s highly influential Code STEMI heart attack program, pioneered by cardiologist Michel Le May, MD.
“It is really all about the team,” said Dr. Le May, Director of the Regional Cardiac Arrest Program. Whether it’s the first responders, regional hospitals or Heart Institute staff, they all have a role to play in helping cardiac arrest patients survive.
Getting the heart beating is only the beginning of the story. Before the patient is stabilized, there is a period where blood is not being pumped throughout the body, which can damage organs, sometimes irreparably. Even without that damage, in 80-90% of cases, going into cardiac arrest means there is some other underlying heart problem that needs to be resolved—and that requires the expertise of the Heart Institute.
While much of the damage to other organs, such as the kidneys, reverses itself or can be reversed through treatment, there is no treatment beyond rehabilitation for damage to the brain. So, that is where the team focuses its efforts.
“There are multiple contributors to brain injury before, during and after a cardiac arrest,” said cardiology resident Juan Russo, MD. At the Heart Institute, the main challenge, he said, is “preventing ongoing brain injury after spontaneous circulation has returned.”
When patients reach the hospital after a cardiac arrest, survival is upward of 50%. But as Dr. Russo pointed out, “Survival is necessary but not sufficient for a good outcome. Neurological recovery is of upmost importance.”
Cooling Is the Key
It has been known for more than a decade that inducing hypothermia can help the body heal and prevent damage from the trauma of cardiac arrest. In fact, defibrillators aside, “Cooling is one of the few treatments shown to improve outcomes,” said Dr. Le May.
The Heart Institute program is the first in Canada to apply the principle in a systematic way, said Program Coordinator Christina Osborne, cooling patients to a target temperature based on scientific consensus about what temperature produces the best outcomes. The program seeks to keep patients in a hypothermic state for 24 hours and then slowly raise their temperature to normal and keep them stable for another day.
“Coming back is a shock in itself,” said Dr. Le May, comparing it to “a boxer still seeing stars.” The major risk, however, is a massive inflammatory reaction, which is detrimental to recovering brain function.

“When we started the cardiac arrest program there were different modes of cooling,” said Osborne. “Now we use internal cooling devices rather than cooling blankets.” When the use of the intravenous cooling device revealed the presence of potentially dangerous blood clots, the protocol was changed to add ultrasound imaging—an abdominal ultrasound on day 3 and a leg Doppler on day 7.
Blood flow to the patient’s brain is assessed using non-invasive technology. This makes truly individualized care possible. Eventually, Dr. Le May said, the technology will evolve to a point where doctors will be able to assess brain activity on a continuous basis. This will help them identify specific areas of the brain with reduced blood flow and help to anticipate functional aspects of day-to-day life where the patient may struggle.
The program started in 2011 as a pilot involving The Ottawa Hospital campuses, and then expanded to all Ottawa hospitals. There was no need to formally roll it out regionally because the other hospitals heard of code ROSC and started using it of their own volition.
“Smaller hospitals might get one Code ROSC patient a year,” said Dr. Le May. “They can’t deal with it, so they will call a Code ROSC protocol.”
“The Heart Institute is the best place for managing somebody who’s had a cardiac arrest. They are some of the sickest patients you will find,” he added. “We have all the resources here. They belong here.”
The Motivation to Press On
For Dr. Russo, focusing on cardiac arrest is an easy choice. It’s a medical condition with high morbidity and mortality and few treatment options. It’s also an “invariably sudden, devastating event for patients and their families,” he said. “Witnessing the medical and social consequences of a cardiac arrest is a strong motivation to work to improve clinical outcomes.”
Now, said Dr. Le May, it’s time for a new goal—to push this program out to other hospitals, so that cardiac arrest patients have the best possibility of full recovery, with intact brain function, wherever it happens.
Providing a Platform for Research
One of the great advantages of the Regional Cardiac Arrest Program is that it provides a unique platform for research, complete with a database of patients who have been subject to the same standardized protocols.
“Very few centres in the world can do this research,” said Dr. Le May.
The overarching question they are trying to answer is how to improve outcomes. The most common cause of death after ROSC is usually brain injury. But why do some patients survive with little or no brain damage, some with extensive brain damage and some don’t survive at all?
The main clinical trial now being conducted, CAPITAL CHILL, is assessing how neurological outcomes differ when a patient receives moderate cooling (31°C) versus mild (34°C). There is no consensus on the best temperature, but current guidelines call for a range between 32 and 36°C.
Dr. Russo is conducting a trial within CAPITAL CHILL called CAPITAL RETURN to assess the clinical significance of non-invasive measurements of cardiac output (the amount of blood being pumped by the heart each minute) and cerebral perfusion (the amount of oxygen being received by the brain). He will present his findings at the Canadian Cardiovascular Congress in October. His abstract has already won an award from the Canadian Cardiovascular Critical Care Society (CANCARE).
It is a great honour for a cardiologist to receive an award for critical care research, said Dr. Le May. Even more important, he continued, is that Dr. Russo’s research has the potential to change how patients—non-cardiac arrest as well as cardiac arrest—are monitored.

